Kruppel-like factor 4 overview
Kruppel-like factor 4
The kruppel-like factors (KLFs) family are zinc finger protein transcription factors, which are important protein in the body and participate in the regulation of cell proliferation, differentiation, embryo development and other important life processes, in cell differentiation and it plays a key role in cell cycle arrest. Kruppel-like factor 4 (KLF4; gut-enriched kruppel-like factor or GKLF) is a zinc-finger transcription factor, and it was first identified in 1996. KLF4 is a member of the KLF family of transcription factors, it is one of the most studied KLFs family members. KLF4 is widely expressed in the body, mainly expressed in the digestive tract, oral cavity, esophageal epithelium, skin epidermis, vascular endothelium and thymic epithelial cells. Among them, gastrointestinal epithelial cells express KLF4 most abundantly, so it is also called GKLF KLF4, also known as gut-enriched kruppel-like factor (GKLF). Which belongs to the relatively large family of SP-1like transcription factors. KLF4 is involved in the regulation of proliferation, differentiation, apoptosis and somatic cell reprogramming. Evidence also suggests that KLF4 is a tumor suppressor in certain cancers, including colorectal cancer.
The Structure and function of KLF4
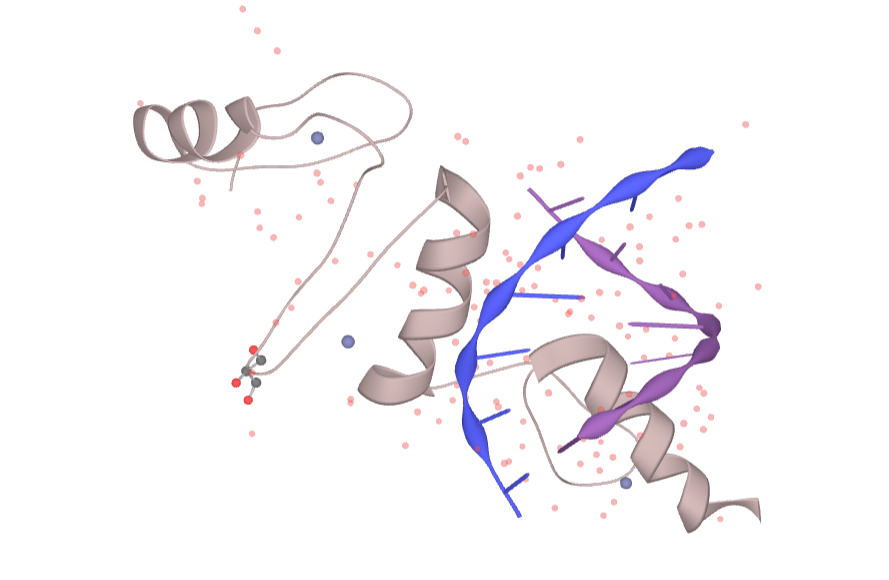 Figure 2. The structure of KLF4
Figure 2. The structure of KLF4
The human KLF4 gene is located on chromosome 9q31 and is a single-copy gene located in the minus strand, covering a 6.3 kb gene segment with five exons. Its encoded protein contains 513 amino acids with a molecular mass of approximately 55 kDa. Its cDNA coding region is 1413 bp in length and encodes a polypeptide consisting of 470 amino acid residues; however, the mouse KLF4 is located on chromosome 4B3, and the mouse KLF4 protein contains 483 amino acid residues in full length, 91% homology with the human KLF4 protein amino acid sequence, and 103 amino acid residues at the carboxy terminus. Analysis by RNA transfer blotting showed that human and mouse KLF4 transcripts were 3.5 kb in length and 53 kDa in relative molecular mass. But KLF4 is expressed higher in mouse tissues. The main feature of KLF4 is the inclusion of three domain: a DNA binding domain, a transcriptional regulatory domain, and a nuclear localization sequence. The KLF4 protein is a transcription factor with a specific binding site, and its recognized DNA motif is 5′-TGGGTGGGGC-3′. This protein contains 3 domains: (1) The DNA binding domain consists of three consecutive C2H2-type zinc finger structures at the carboxy terminus;(2) Nuclear localization sequence—–there are two independent nuclear localization sequence, and both nuclear localization sequences can independently and efficiently transfer KLF4 into nucleus; (3) The transcriptional regulatory domain is located at the amino terminus and comprises a transcriptional repression domain.
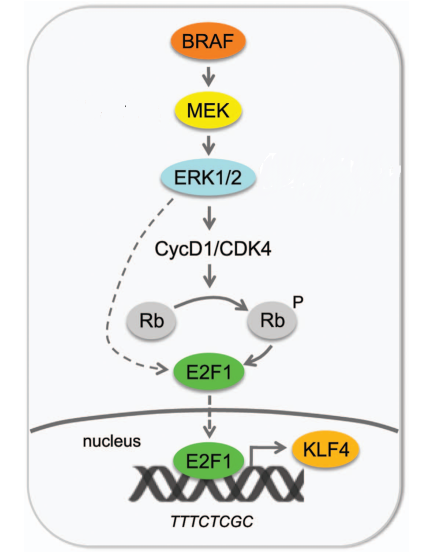 Figure 3. The regulation of KLF4 signaling pathway
Figure 3. The regulation of KLF4 signaling pathway
E2F1 acts downstream of ERK1/2 to regulate the expression of KLF4 by direct binding to KLF4 promoter. ERK1/2 can regulate E2F1 via CycD1/CDK4, which in turn promotes phosphorylation of pRB and consequent release of E2F1. Alternatively, ERK1/2 can directly or indirectly regulate E2F1 expression. Previous studies suggest that the induction of KLF4 expression is mediated by the transcription factor E2F1, which binds the KLF4 promoter (TTTCTCGC) in a binding site that differs from the canonical E2F1 sequence for the presence of a thymine instead of Cytosine in position 5th. The biological relevance of the regulation of KLF4 by E2F1 is supported by a positive correlation between the expression of KLF4 and E2F1 in melanoma tissues. First, mutated BRAF increases CDK4/CyclinD1-dependent pRB phosphorylation, and this effect is mediated by ERK1/2. It is, therefore, reasonable to assume that phosphorylation of pRB leads to its inactivation, with consequent destabilization of the pRB/E2F1 interaction, and release of E2F1, which is free to induce transcription of KLF4. Second, ERK1/2 modulates the expression of E2F1, as indicted by a strong reduction of E2F1 protein level upon MEK1/2 inhibition. At present it remains unclear whether ERK1/2 modulates the expression of E2F1 protein directly, since ERK1/2 has also a non-catalytic activity, or indirectly through a third protein, which, in turn, modulates E2F1.
In addition, another KLF4 signaling pathway involving TGF-β1 is also worth mentioning. Proposed model for KLF4-mediated link between TGF-β1-induced gene transcription and histone H3 acetylation. In basal conditions, KLF4 is physically associated with PTEN and dephosphorylated by it in VSMCs. Upon TGF-b1 stimulation, KLF4 dissociates from PTEN, thus resulting in KLF4 phosphorylation and subsequent association with p300. KLF4 acts as a transcription factor by recruiting p300 to the KLF4 binding site of TGF-b1-regulated target promoters and initiating gene transcription. As shown in the following figure.
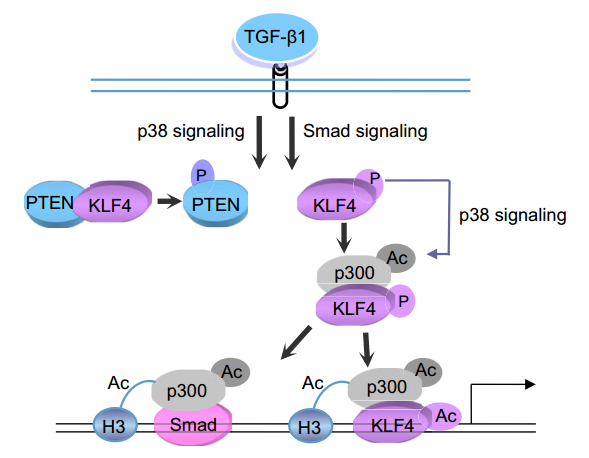 Figure 4. KLF4 signaling pathway involving TGF-β1
Figure 4. KLF4 signaling pathway involving TGF-β1
Role in diseases
Several lines of evidence have shown that KLF4 role in disease is context dependent where under certain conditions it may play one role and under different conditions it may assume a complete opposite role. KLF4 is an anti-tumorigenic factor and its expression is often lost in various human cancer types, such as Colorectal cancer, gastric cancer, esophageal squamous cell carcinoma, intestinal cancer, prostate cancer, bladder cancer and lung cancer. However, in some cancer types KLF4 may act as a tumor promoter where increased KLF4 expression has been reported, such as in oral squamous cell carcinoma and in primary breast ductal carcinoma. Also, overexpression of KLF4 in skin resulted in hyperplasia and dysplasia, which lead to the development of squamous cell carcinoma. KLF4 plays an important role in several vascular diseases where it was shown to regulate vascular inflammation by controlling macrophage polarization and plaque formation in atherosclerosis. It up-regulates Apolipoprotein E, which is an anti-atherosclerotic factor. It is also involved in the regulation of angiogenesis. It may suppress angiogenesis by regulating NOTCH1 activity, while in the central nervous system its overexpression leads to vascular dysplasia. Similar finding in esophageal epithelium was observed, where overexpression of KLF4 resulted in increased inflammation that eventually lead to the development of esophageal squamous cell cancer in mice. KLF4 is essential for the cellular response to DNA damage. It is required for preventing cell cycle entry into mitosis following γ-irradiation-induced DNA damage, in promoting DNA repair mechanisms and in preventing the irradiated cell from undergoing programmed cell death (apoptosis). In one study, the in vivo importance of KLF4 in response to γ-irradiation-induced DNA damage was revealed where deletion of KLF4 specifically from the intestinal epithelium in mice lead to inability of the intestinal epithelium to regenerate and resulting in increased mortality of these mice.
References
- Sun H, Peng Z, Tang H et al. Loss of KLF4 and consequential downregulation of Smad7 exacerbate oncogenic TGF-β signaling in and promote progression of hepatocellular carcinoma. [J] .Oncogene, 2017, 36(21): 2957-2968.
- Riverso M, Montagnani V, Stecca B. KLF4 is regulated by RAS/RAF/MEK /ERK signaling through E2F1 and promotes melanoma cell growth. [J]. Oncogene, 2017, 36(23): 3322-3333.
- Alejandre Alcazar Miguel A, Kaschwich Mark, Ertsey Robert et al. Elafin treatment rescues EGFR-Klf4 signaling and lung cell survival in ventilated newborn mice. [J]. Am. J. Respir. Cell Mol. Biol., 2018.
- Xu Lijun, Zheng Lili, Wang Zhifang et al. TNF-α-Induced SOX5 Upregulation Is Involved in the Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells Through KLF4 Signal Pathway. [J]. Mol. Cells, 2018, 41(6): 575-581.
- Human mucin 1 oncoprotein represses transcription of the p53 tumor suppressor gene. “Wei X., Xu H., Kufe D.Cancer Res. 2007, 67:1853-1858.

